The application of the Heart Team to facilitate TAVR in patients with aortic stenosis is now established in routine clinical practice with well-defined patient pathways. It is appealing to consider that the application of this model to patients with degenerative mitral valve (MV) disease would be equally efficacious. Although up to 10% of patients above the age of 75 years have significant MR, only 15% undergo surgical treatment, This suggests that a substantial group of patients may be eligible for transcatheter mitral valve therapies (TMVT). Despite this, the number of patients being referred for TMVT are small and screening failure rates remain high. This advocates an alternative strategy to best identify and treat these patients. In the following report we detail our 12-month experience in delivering a dedicated Transcatheter Mitral Valve Assessment (TMVA) clinic.
A one-stop TMVA clinic was run by Cardiologist (RR) with 10 years’ experience in managing patients with heart failure and structural imaging (echocardiography and cardiac CT). Over the 12-month pilot period there were 77 referrals for consideration of TMVT. 23(30%) referrals originated from the Cardiothoracic Surgical team, 34(44%) from External Cardiologists, and 20(26%) from Internal referrals.
At our institute, the TMVT toolbox was comprised of TMV replacement with: 1) an Intrepid (Medtronic, MN, USA) or Sapien-3 (Edwards Lifesciences, CA, USA) valve for cases of mitral-annular-calcification (MAC), 2) MitraClip (Abbott, IL, USA) edge-to-edge repair for degenerative or functional MR and 3) indirect annuloplasty with an ARTO (MVRx, CA, USA) device. Upon receipt of the referral and the echocardiographic images one of two “one-stop” clinic pathways were adopted. Where edge-to-edge repair was under consideration, a detailed review of the current heart failure therapies was undertaken along with the patient’s symptomatic status. If a patient was sub-optimally treated for heart failure or was minimally symptomatic, further advice to the referrer was provided and re-referral recommended if required. Where a patient met clinical criteria for edge-to-edge repair same day transoesophageal echocardiography (TEE) was performed. For patients being considered for an alternative TMVT, the TEE was substituted for a same day multiphase ECG-gated cardiac CT-scan. This was firstly reviewed for device and patient suitability before determining the need for a TEE. The pathway included access to bioengineering and computational fluid dynamics to assess suitability for valve-in-MAC and TMVR cases [3, 4].
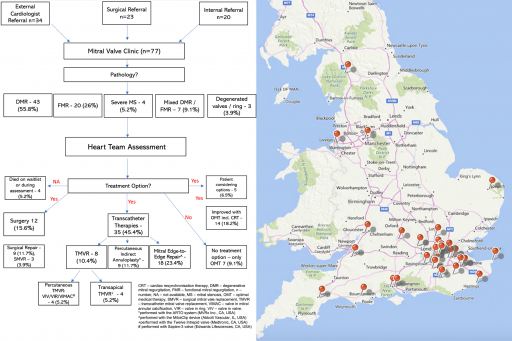
Once all assessments were completed, the case was discussed with the “Heart Team” that included mitral valve surgeons, heart failure specialists, imaging specialists and interventional cardiologists to sanction further treatment. The patient was subsequently scheduled for the relevant TMVT.
Over a 12-month period, 77 patients were evaluated in the clinic. The mean age was 78.3 years, of whom 45 (58.4%) were men, with 67 (87%) patients reporting NYHA class III-IV symptoms. This was a high-risk cohort with a mean Euroscore-II of 5.72%, an STS-score for mortality 5.8%, and 25 (32.5%) having had previous cardiac surgery. Echocardiography revealed grade III-IV MR in 71 (92%), with 27 (35.1%) of patients having a left ventricular ejection fraction of ≤ 35%. Dedicated same day cardiac MDCT was performed in 36 (46.8%) patients and same day TEE in 41 (53.2%) patients. The aetiology of MV disease and outcomes is detailed in the figure. Treatment was delivered to 47(61%) patients. Of these 12(16%) who were initially expected to be high-risk for surgery were ultimately accepted for surgical treatment. A further 14(18%) patients were declined further treatment owing to minimal symptoms and scope for further medical optimisation. In 7(9%) patients who met clinical criteria for treatment, there were no TMVT options available following appropriate imaging assessment (small LV cavity, risk of LVOT obstruction, degree of MAC). 4 (5%) patients died whilst awaiting a TMVA clinic appointment or treatment.
There are a number of important observations from our experience with a dedicated TMVA clinic. Firstly, in our TMVA model, we positioned a heart failure and imaging specialist as the entry point to the pathway with same day diagnostics for the majority of patients. We believe this bestowed greater referrer confidence to the clinic and increased referrals to the service. Secondly, the incorporation of MV surgeons earlier in the pathway, increased surgical confidence in the TMVA clinic. This enabled bilateral referral pathways to be established with not only MV surgeons operating on patients initially deemed to be at high surgical risk, but also patients being referred to the TMVA clinic who were not ideal for surgery. Finally, the positioning of an imaging specialist at the fulcrum of the pathway enabled quicker decision making to be accomplished and facilitated access to bioengineering solutions for pre-procedural planning and ultimately device deployment.
In patients with MV disease being considered for TMVT, novel approaches to the Heart Team dynamics may enable greater access to treatment and improved patient outcomes. Further evaluation of TMVA clinic models is indicated to confirm this hypothesis.